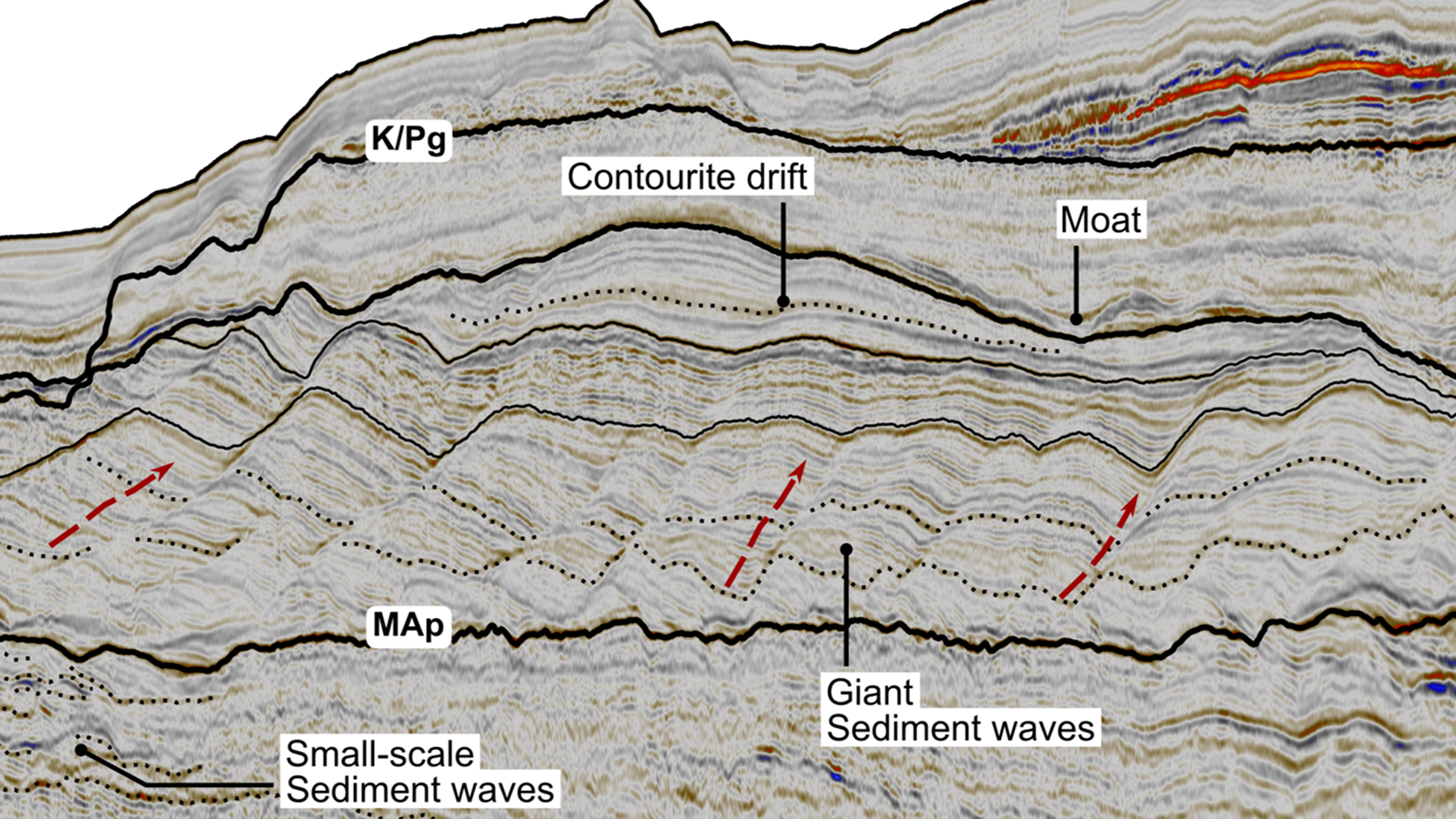
When you purchase through link on our website , we may realize an affiliate commission . Here ’s how it work .
For the first prison term , the U.S. Food and Drug Administration ( FDA ) has authorized the marketing of nicotine pouches , synthetic packs of nicotine designed to be place under a individual ’s lip .
In a statementpublished Jan. 16 , the means stated that 20 different Zyn nicotine pocket products can now be marketed to adult eld 21 and over . That does n’t mean the sack arenow " FDA approved " as drugs intended to handle disease would be , nor that they are safe . Rather , the pouch have meet the FDA ’s criteria for nicotine - based product that can be sold to the public .

Twenty types of Zyn nicotine pouches can now be legally marketed in the U.S. to adults ages 21 and over, the FDA says.
After an " extended scientific revaluation , " the FDA said the pouches have met thepublic health standardset by the 2009 Family Smoking Prevention and Tobacco Control Act , which weighs the overall risk and benefits that such products would pose to the general population . The federal agency notes that the pouches contain lower levels of harmful substances than cigarettes do and thus pose a downhearted risk ofcancerand other serious health issues .
However , some public wellness and aesculapian organisation take that by empower the marketing of these products , the FDA has ignored their warningsabout how serious the product could be to new hoi polloi . Meanwhile , other experts have monish that there is not yet enough scientific grounds to fend for their use as a smoke surcease peter . The FDA ’s statement suggests that the pouches could be helpful in the event that people who smoke completely switch to the pouches instead .
Here ’s everything we cognise so far about nicotine pouches and the Modern FDA authorisation .

People use nicotine pouches by placing them between their lip and gums.
Related : Simple vaping ' quitline ' can facilitate over 40 % of young people quit , study ascertain
What does the FDA authorization mean?
The fresh FDA authorization means that 10 flavors of Zyn nicotine pouches can now be legally marketed in the U.S. to adults ages 21 and over . These 10 flavor come in two nicotine Department of State : 3 and 6 mg . The company markets these flavor as chill , cinnamon , citrus fruit , coffee berry , cool mint , menthol , peppermint , fluent , spearmint and ground-berry .
In its Jan. 16 statement , the FDA notes that this latest authorization does not intend that these products are safe for use or that they are " approved " by the agency . This is as opposed to several types of nicotine - refilling therapies , including " nicotine gum " and " nicotine patches , " whichhave been approved by the FDA as quit - smoking help . These latter products have been prove aremuch less addictive than butt , do n’t cause decease or disease and are usually only used temporarily .
Products like Zyn do n’t need to be prove good and good for a specific population — the manufacturer just necessitate to comply with " demand under the law to lend its production to market , " the FDA website says . In addition , the FDA assesses whether a given mathematical product would offer more benefit than risks to the general population , consideringthe universe as a whole , including users and nonusers of tobacco mathematical product . It weighs , for illustration , whether the great unwashed who currently smoke might benefit from switching to the new mathematical product , or if nonusers might be likely to jump using nicotine for the first time once the raw product hits the market place .

Questions remain about whether nicotine pouches could help adults quit smoking.
What are nicotine pouches?
Nicotine pouches like Zyn are small , rectangular bags of powder that are placedbetween a person ’s sassing and gums to deliver nicotine . Theydon’t take any of the tobaccofound in formal smoke products , such as cigarettes and cigar . Nicotine from the pocket enters the blood stream and makes its way to thebrainvia the oral mucosa — thewet , soft tissues that line the oral cavity .
" Nicotine pouches are a comparatively new category of tobacco products that are unique because they contain a crystallized nicotine pulverization instead of tobacco leaf,“Tory Spindle , an associate prof of psychopathology and behavioral science at Johns Hopkins Medicine , secernate Live Science in an e-mail .
" This intend that they by and large turn back fewer harmful element compared to traditional tobacco products , " he enounce . product like fag contain both tobacco and a variety of additives , both of which contain carcinogens .

But nicotine pocket are n’t costless of harmful chemical . " They do still bear nicotine which is an addictive heart as well as other substances such as flavorants , " Spindle allege . A survey published in the journalTobacco Controlin 2022 retrieve that 26 of 44 sample nicotine pocket products check cancer - causing chemical substance known astobacco - specific nitrosamines . The nicotine in the pouches can be derive from tobacco industrial plant extract , explaining how these chemicals stop up in the products .
The same study also found that each of the try out pouches check between 1.79 and 47.5 mg of nicotine . By compare , fag contain an estimated6.17 to 12.65 mgand e - fag release about0.5 to 15.4 mgper 300 puffs , while nicotine patches or gum releaseup to 21 mgor4 mg of nicotine . So , depending on the brand , nicotine pouches still deliver substantial amounts of nicotine compared with other products on the market place .
Can nicotine pouches help people quit smoking?
For a ware to get marketing authorization , there call for to be sufficient evidence that it offers great benefits than risks to the health of the general population , Matthew Farrelly , director of the Office of Science in the FDA ’s Center for Tobacco Products , say in the agency ’s Jan. 16 financial statement .
" In this case , the data show that these nicotine pocket products meet that streak by do good adults who use cigarettes and/or smokeless tobacco products and totally switch to these intersection , " Farrelly said .
However , experts are unconvinced that there is enough scientific evidence to support the idea that adult smoking car will actually make the switch .

Related : Nicotine vapes are one of the good tool to help people quit smoke , revue of 300 trial suggests
" There are some anecdotal reports of people switch from smoking or using traditional oral tobacco products such as ' dip ' or ' chew ' to nicotine sac , " Spindle said . " But the gilded stock research subject area require to answer this question ( randomized controlled tryout ) have not been done . "
Randomized controlled trials allow scientists to at once assess whether one constituent causes another . In this suit , such a trial would likely involve a large group of people who currently smoke , a pct of whom would be give nicotine pouches to see whether they ’re more likely to quit than people given a dissimilar intervention or nothing at all .

One late scientific review article , publish in 2024 in the journalExperimental and Clinical Psychopharmacology , suggested that oral nicotine pouch may be a " practicable harm reduction " option for citizenry who switch over completely from formal baccy products . However , that review also concluded that randomized assure trials are ask to determine if people will continue to use the pouches in the long condition . More sovereign inquiry on the topic , not funded by the tobacco industry , is also need , the review article tell .
What are the potential risks of using nicotine pouches?
Nicotine pouch purpose is tie in with several health effects , include addiction to the nicotine within them , Spindle said . These effect can be in particular pronounced for younger mass , who may still use the product despite the unexampled authorization only allowing selling towards over-21s .
" Developing an addiction to a substance can have negative impacts on the developing mastermind , which is why it is important for kids not to use them , " Spindle order . Exposure to nicotine during adolescence canalter signaling between neuronsin the prefrontal cortex of the brain . This part of the wit insure an array of cognitive affair , including determination - making and reasoning , and it’sone of the last regions of the brain to suppurate .
Nicotine pouches are also known to get side effects , such asnausea and mouth sore . Because they do n’t let in all of the harmful substances found in traditional tobacco plant products , some hoi polloi might assume that the pouches have fewer long - term health effects . However , that ’s not recognise for indisputable , andtheir impact on unwritten healthis especially unclear , Spindle sound out .

The FDA say it will " closely monitor the marketing and use of these products , " harmonize to the Jan. 16 argument . This monitoring will include ensuring that the marketing of the patches online , on tv set and on the receiving set is aim to grownup ages 21 and over .
But several system have raised concerns about whether this level of regularisation will be enough to curb the use of nicotine pouches by younger groups . The look of authorized Zyn productsare likely to attract to this demographic , the governance noted .
" While it has not been sold in the U.S. for long , we are already seeing very concerning levels of youthfulness use [ of Zyn ] , and troubling use of influencers and fame to promote the merchandise , " the American Lung Associationsaid in a program line released Jan. 16 .
— Is vaping intelligent than smoking ?
— Quitting smoking by age 35 brings your risk of decease in line with ' never smokers '
— FDA fair game mint- , yield - flavored e - butt to protect young vapers

A 2024 National Youth Tobacco Survey suggested thatonly 1.8 % of middle and high school student in the U.S. apply nicotine pouches . However , they were still the secondly - most commonly used tobacco product after einsteinium - cigarettes in this demographic .
Another subject field , published in the daybook JAMA in 2024 , found that sale of nicotine pouches in the U.S. hadincreased by 641 % between 2019 and 2022but that only 2.9 % of adults age 18 and up report ever using them . This raised questions as to what other group have been using and grow the demand for these product .
This clause is for informational purposes only and is not meant to offer medical advice .