When you buy through links on our site , we may clear an affiliate commission . Here ’s how it works .
The driving force to eat may be controlled by a very simple circuit in the learning ability , newfangled research in mice suggest .
Just three types ofbraincells collaborate to suppress or heighten appetite , driving mouse to rust less or more food , the work found .

A new study suggests that appetite may be controlled by three types of neurons.
First , specialised neurons detect " thirst - point hormones " that say whether an animal is full or hungry . These neuron then control the activity of neurons in a unlike part of the Einstein , which in turn , controls a third band of nerve cell in the jaw : These last nerve cells direct the drive needed for jaw .
The three - part circuit act like a inborn reflex , without needing conscious sentiment to maneuver it — like to yanking your bridge player by from a hot aim . In this case , the stimulus that set off the reflex is a hunger - signaling hormone , and the lead action is moving the jaw to jaw .
Related:‘This is largely unmapped district ' : Scientists reveal the brain ’s ' veneration tour ' works differently than we thought
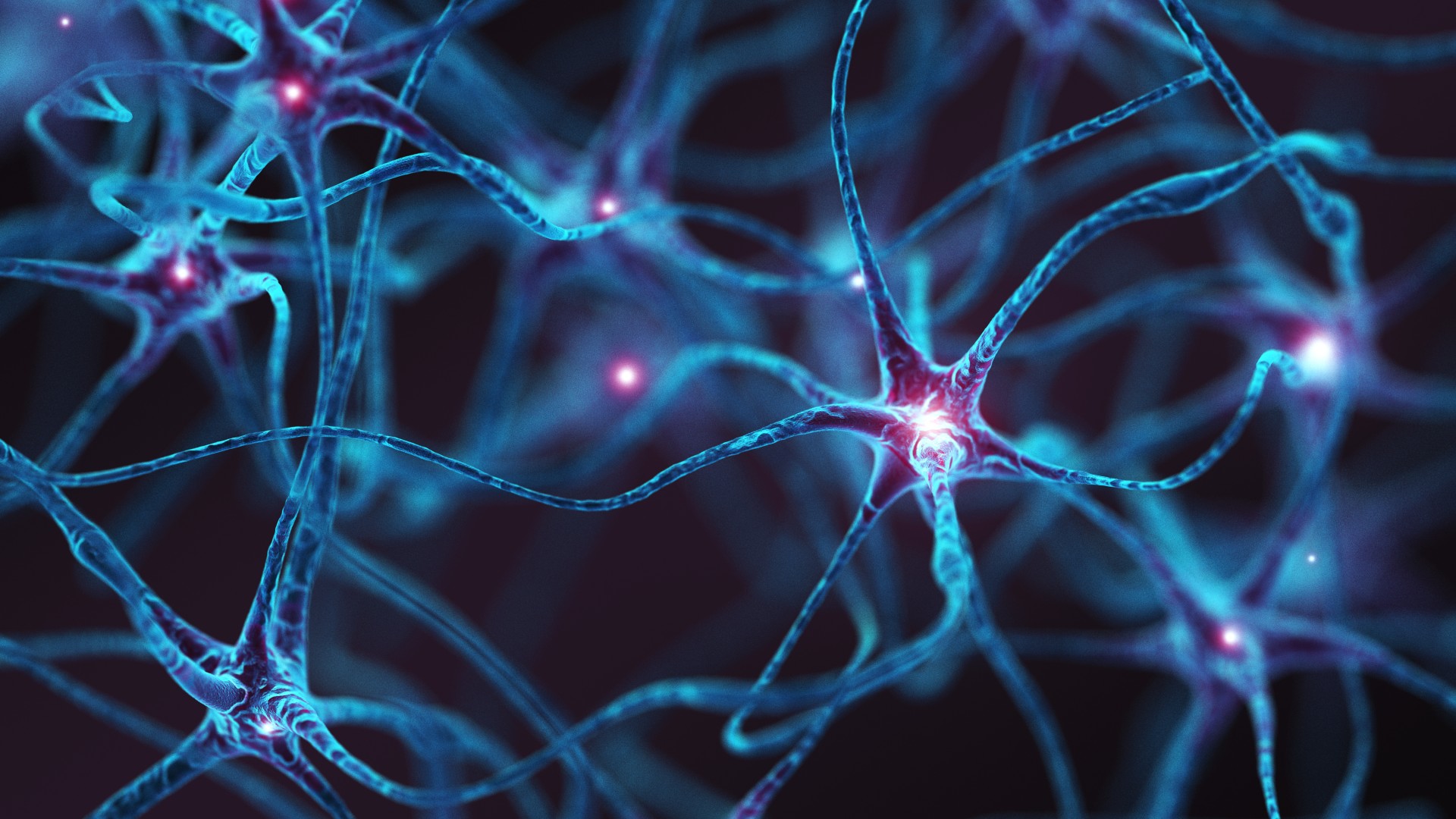
Neurons within the hypothalamus region of the brain belong to the circuit that regulates appetite, the study authors found.
The newfangled study , published Oct. 23 in the journalNature , include only mice , and the three - part racing circuit has yet to be identified in humans . However , if it is found in people , this find could " change the narrative " on obesity , the generator behind the study debate .
" The control of how much we eat and when we exhaust is not so much found on your decision process , it just happens — it ’s a mere circuit,“Christin Kosse , lead study source and a research associate at The Rockefeller University in New York , say Live Science .
In recent decades , medical governing body have characterizedobesity as a inveterate disease with an array of suit , include genetics . Previously , it was consider to be just a outcome of personal decisions around eating . The newfangled study adds evidence to the idea that physiological difference of opinion underlie obesity .
The new research also helps wed a identification number of survive theories about hunger together .
The first is known as " set full point hypothesis . " Some scientists remember that people generally have a body weighting mark point determined by their genetic science and their surround . As the hypothesis goes , a person ’s dead body endeavor to keep weight constant , even if they eat more or less food than they need — as such , there are various physiologic mechanics that help conform for these fluctuations in calories .
But if these mechanism break down , people may gain or fall behind weight .

For instance , after a filling meal , fertile prison cell and cells in the gut outlet hunger - controlling hormones that signal to the brainiac that you should stop eating . These signals kick inapproximately 20 minutesafter you eat enough to be overeat . However , if this internal secretion signaling is break up for some understanding , hoi polloi can become highly hungry even when they ’ve consume enough . In some cases , this can drive people to overeat to the full stop ofbecoming severely obese .
old research has prove that neurons in part of the brain called the hypothalamus diddle a persona inregulating appetence , and they are targeted byweight - loss drugs in the same grade as Ozempic . In addition , reductions in a protein call brain - derived neurotrophic factor ( BDNF ) in the brainare associated with obesity in both animals and humans .
The fresh study connects the dots between these determination .

In their experiments , Kosse and colleagues discovered that neurons in the hypothalamus that make BDNF are activated in the brainiac of mice who became obese after being feed a high - blubber diet . This implies that these BDNF - get neuron trip in response to weight gain in ordination to inhibit the rodents ' appetites .
To test this hypothesis , the squad switched these BDNF nerve cell on and off . When the neurons stopped working , the black eye ate about 1200 % more nutrient than they normally would , and they made chewing crusade even when there was n’t any solid food available to them . In some font , they even commence attempting to eat object , such as wooden block , suggest that this reply was automatic .
Conversely , when the neurons were turn on , the opposite effect happened : The mice stop over eating and also did n’t make any masticate movement . Once the team realized these BDNF neurons flip out a key electrical switch in the mastermind , they identified the two other types of nerve cell involved in this circuit .
— ' Flow state ' uncovered : We finally know what happens in the wit when you ’re ' in the zone '
— Super - elaborate single-valued function of mental capacity cells that keep us awake could improve our understanding of consciousness
— A deep brain web may underlie many psychiatric disorders

Kosse believe that it is likely that we man have a corresponding controller system for hunger in our brain .
move forward , the team now wants to see if this circumference change in the context of dissimilar emotional State , such as anxiousness .
Ever wonder whysome people build up musculus more easily than othersorwhy freckles come out in the sun ? beam us your questions about how the human body works tocommunity@livescience.comwith the dependent argument " Health Desk Q , " and you may see your question answered on the web site !