When you purchase through link on our site , we may realize an affiliate commission . Here ’s how it works .
The world ’s first treatment that usesCRISPRgene - editing technology has been okay .
Exa - cel , also known by its brand name Casgevy , get its firstregulatory approvalon Nov. 16 , 2023 from the U.K. Medicines and Healthcare products Regulatory Agency ( MHRA ) to treat two debilitating roue disorderliness : sickle - cell diseaseandtransfusion - dependent beta - thalassemia . The U.S. Food and Drug Administration ( FDA ) subsequently approved the therapy as a discourse forbothdisorders .
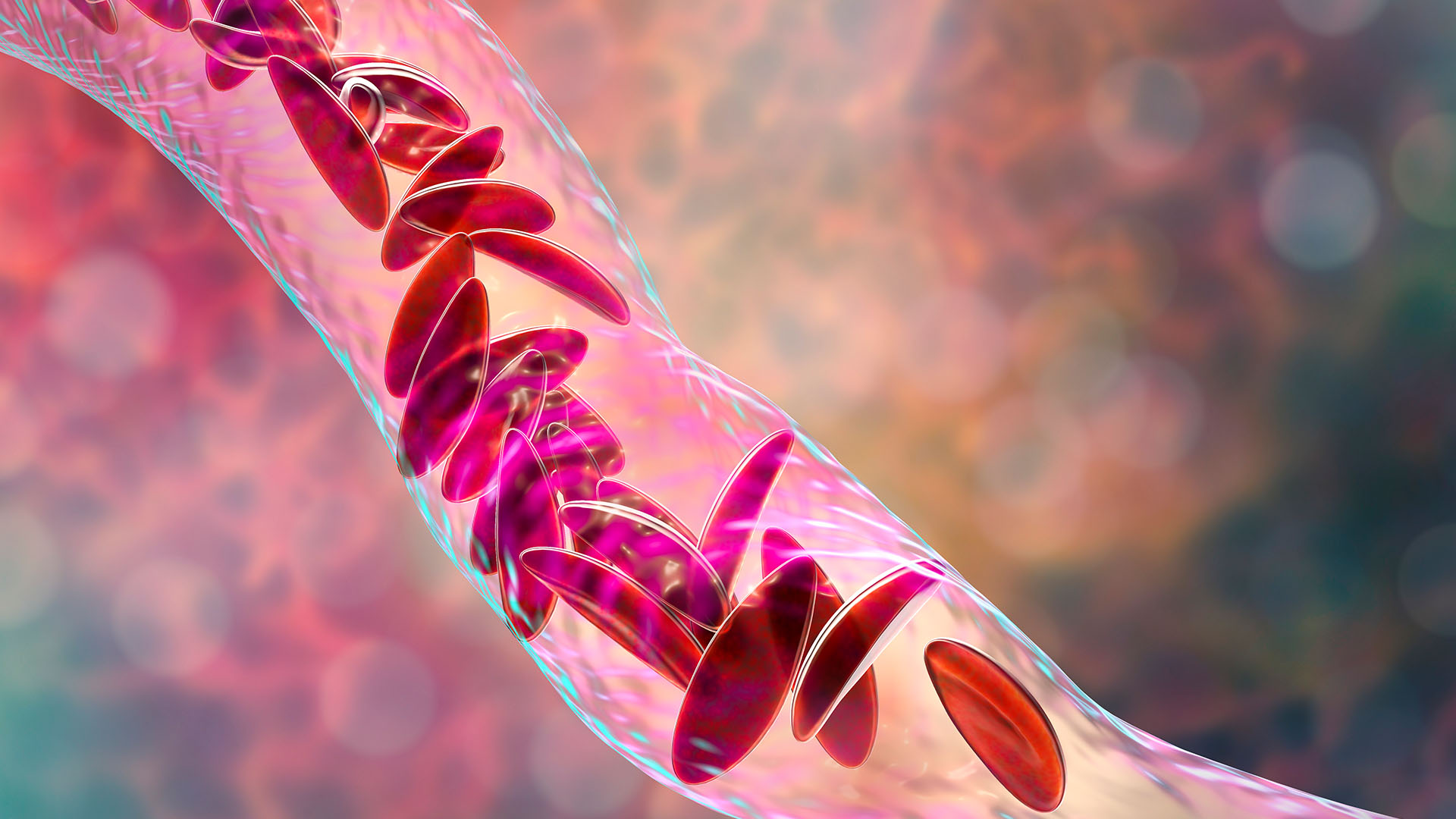
Sickle-cell disease causes red blood cells to become C-shaped and sticky, so they clog up blood vessels.
The regulators ' historic decisiveness to approve Casgevy may signal the starting signal of a raw era ofgene therapy . However , questions remain surrounding the treatment ’s affordability and its long - term safety .
Here ’s what we live so far about Casgevy .
Related : A teen ’s cancer is in absolution after she meet new cells edited with CRISPR

In patients with sickle cell disease, some of their red blood cells are crescent-shaped and are unable to carry oxygen around the body as efficiently.
What does the first approved CRISPR therapy treat?
The MHRA approved Casgevy to plow sickle - cell disease ( SCD ) and blood transfusion - dependent genus Beta - thalassaemia . These are lifelong , hereditary disorder make by mutant in the gene that code for hemoglobin , a protein that crimson blood cells require to channelise oxygen around the body .
More than 100,000 peoplein the U.S. are figure to have SCD , but the rate are high for some populations than others . For example , 1 in every 365 Black babies is born with SCD . The disease vary the shape of a mortal ’s red blood jail cell so that they become one C - shape , rather than round . The reaping hook - comparable cell croak apace and also stick to each other , blockingblood vessels . As a result , patient developanemiaand often get bouts of severe bother calledpain crisis .
Beta - thalassemia affects around1 in 100,000 peopleworldwide , and it disproportionately affects those ofMediterranean , Asiatic , African and center Eastern blood line . patient with beta - thalassaemia do n’t produce enough haemoglobin , which can lead to grievous anemia , whereas sickle - prison cell anemia stems from a lack of healthy ruddy blood cells . " Transfusion - dependent " have in mind that the disease is so severe that patients must haveregular red rip cell transfusionsthroughout their lives .

This illustration of CRISPR in action shows Cas9 (in blue and pink) attached to the DNA (in purple) alongside the guide RNA molecule (in orange).
How does Casgevy work?
Casgevy is found on a revolutionary gene - redaction proficiency called CRISPR which wasfirst developed in 2012 .
The CRISPR system of rules reduce genes out of DNA using an enzyme called Cas9 . These " molecular scissors " are guided to target DNA by a particle ofRNA . The engineering was adapted from a born defence mechanism mechanism thatbacteriaand other simple organism called archaea use against computer virus .
Casgevy aim a gene calledBCL11A. The gene code for a protein that would normally regularise theswitch from the fetal version of hemoglobinto the adult variation short after parturition . However , in patient with SCD and beta - Mediterranean anaemia , the grownup hemoglobin is defective .

The goal of Casgevy is to disable BCL11A and thus allow the body to keep making fetal hemoglobin , since the adult interlingual rendition does n’t work . To do this , roue - making stem cells are taken from a patient ’s osseous tissue marrow and the BCL11A gene is edit using Casgevy in the lab . The new - modify cells with functioning hemoglobin are then impregnate back into the affected role ’s body . Before the extract , the patient must take a chemotherapy drug called busulfan to eliminate the unedited cells still in their bone marrow , STAT News reported .
This process of adjusting to the fresh , edited cellphone is protracted . " Patients may need to expend at least a month in a hospital facility while the treated cadre take up residence in the os marrow and begin to make red line of descent cells with the static form of haemoglobin , " the MHRA suppose in astatement .
In two late - stage clinical trials , Casgevy bushel haemoglobin production in most patients with SCD and genus Beta - thalassemia and alleviate their symptoms . Twenty - eight out of 29 patients with SCDdidn’t see any knockout pain crises for at least a year after being treated with Casgevy . Similarly,39 out of 42 patientswith beta - thalassemia did n’t call for cherry blood electric cell transfusion during the same post - treatment time period . The remaining three patients were more than 70 % less potential to need a transfusion .

Is Casgevy safe?
No serious safety concern were flagged in either of the two tardy - degree clinical test of Casgevy , although some transient side effects , such as fever and fatigue were reported . Both of these trials are on-going and Casgevy ’s long - term safety continues to be monitored by regulatory soundbox , such as the MHRA and the FDA , and by the therapy ’s manufacturers , Vertex PharmaceuticalsandCRISPR Therapeutics .
However , there are still some concerns about the base hit of CRISPR - base therapy , in world-wide . Namely , there ’s concerns about " off - target " consequence , which fall out when Cas9acts on other part of the genomethat were n’t mean to be changed and make unwanted side effects .
" It is well hump that CRISPR can result in spurious genetic limiting with strange consequences to the treated cells,“David Rueda , chair of Molecular and Cellular Biophysics at Imperial College London , recite theU.K. Science Media Centre . " It would be substantive to see the whole - genome sequencing data for these cell before coming to a conclusion , " he said . This would involve survey all the desoxyribonucleic acid in Casgevy - edited cells to see if there are any off - object effects .

Related:2 women bring in Chemistry Nobel Prize for gene - editing cock CRISPR
Where has Casgevy been approved?
In November 2023 , the U.K. approve Casgevy for peopleover the years of 12with either sickle - cell disease or blood transfusion - dependant genus Beta - thalassaemia . In December , the FDA approved the treatment for masses ages 12 and elderly with sickle - cell disease and in January 2024 , the agency okay Casgevy for people with blood transfusion - dependent genus Beta - thalassemia belong to the same eld category .
When will Casgevy be available to patients?
It ’s unclear when Casgevy will become available , but its reach will mostly depend on its toll . Gene therapy can costmillions of dollarsand it looks like Casgevy will be no exception . This could make it inaccessible for many citizenry who need it .
" The challenge is that these therapies will be very expensive so a means of making these more accessible globally is key,“Kay Davies , a prof of anatomy at the University of Oxford , told the U.K. Science Media Centre .
Vertex has yet to set a price for Casgevy in the U.K. , a spokesperson from the companytold Nature , but is " working with the wellness authority to secure reimbursement and access for eligible patients as quickly as possible . "

What other CRISPR therapies are in development?
Intellia Therapeuticsis formulate CRISPR therapies to regale inherited disease from inside the torso , STAT News reported .
— CRISPR used to ' reprogram ' cancer cadre into sound musculus in the laboratory
— Meet ' Fanzor , ' the 1st CRISPR - corresponding system found in complex life

— CRISPR - edited juicy shrank neoplasm in mice . Someday , it could work in citizenry , scientist say .
In addition , a tweaked version of CRISPR called " root redaction " that can point the individual construction blocks ofDNAis being tested as a way to treat disease . For representative , Verve Therapeutics is screen such anexperimental treatment for heart disease . Another prognosticate novel type of therapy , call " prime editing , " involves CRISPR but also " incorporates extra enzymes and genetic direction to introduce , delete or rewrite short segment of DNA , " STAT News describe .
This article is for informational design only and is not meant to offer aesculapian advice .

Ever wonder whysome people progress muscle more easily than othersorwhy lentigo come out in the Lord’s Day ? Send us your question about how the human consistency act tocommunity@livescience.comwith the subject course " Health Desk Q , " and you may see your question reply on the website !
Legendary ' cleaning lady of the ocean ' in South Korea freedive well into their fourscore . A young field of study hints at how .
What is xeroderma pigmentosum ? The rarefied genetic upset that storm the great unwashed to avoid sun

What are neural processing unit ( NPUs ) and why are they so crucial to modern computer science ?



