When you purchase through link on our internet site , we may earn an affiliate commission . Here ’s how it works .
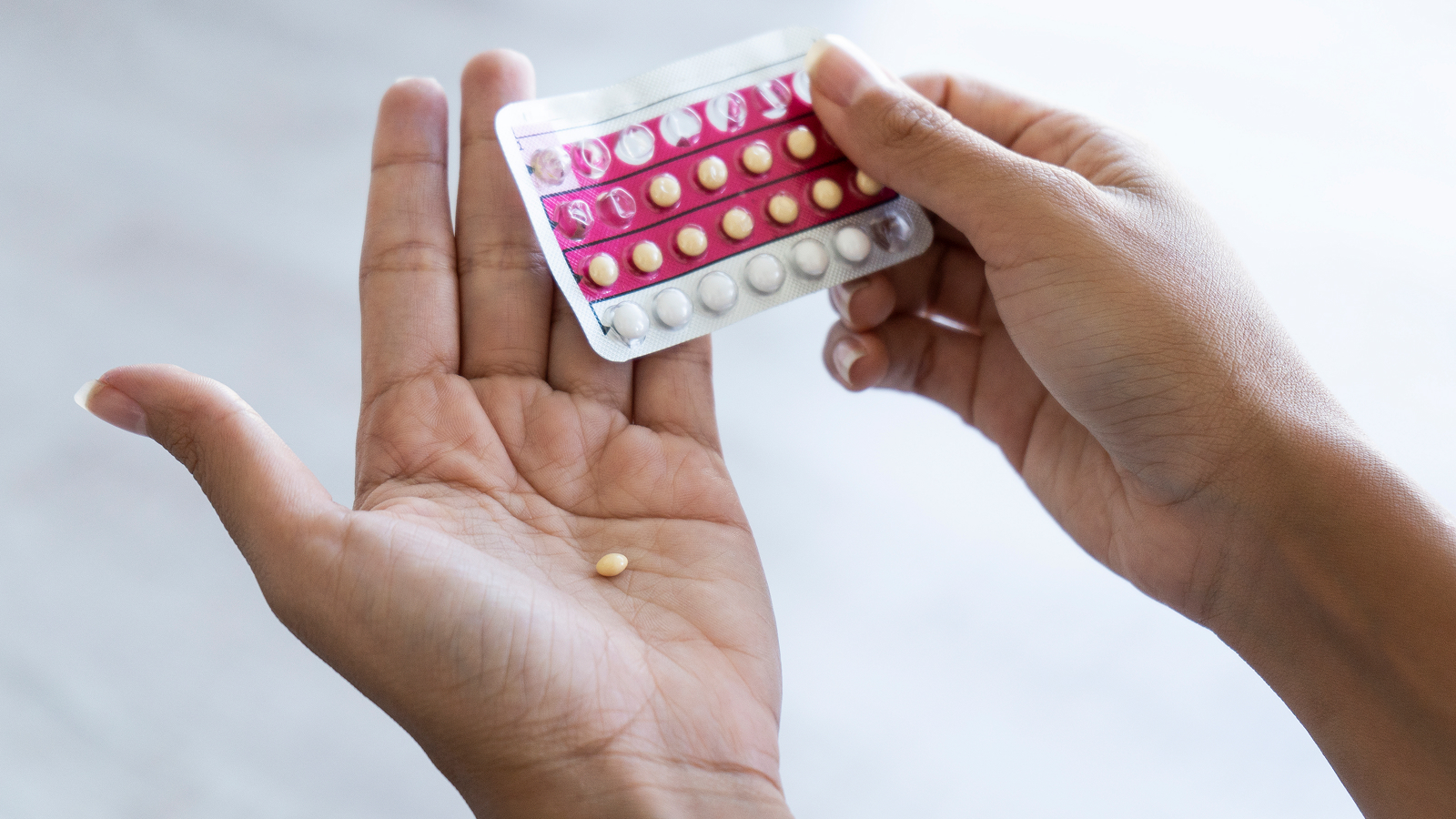
In a consentaneous ruling , the Supreme Court of the United States ( SCOTUS ) has rejected a bid to curtail people ’s access to the wide usedabortionpill mifepristone . The drug is one half of a two - pill regime prescribed formedication abortion , which made upmore than 60 % of all abortionsin the U.S. last class .
The complainant in the case were members of the Alliance for Hippocratic Medicine ( AHM ) , a collective of anti - abortion groups that includes doctor . The defendant was the Food and Drug Administration ( FDA ) .

SCOTUS has issued a highly anticipated ruling about whether an anti-abortion group had grounds to sue the FDA over its regulation of an abortion pill.
The plaintiffs argued that AHM has the rightfield to sue the FDA over how it regulatesmifepristonebecause the collective ’s members may terminate up treating patients who know side effects of the drug . In recent age , the FDA has made it easier for patient to get at the birth control pill — for instance , multitude can now be dictate mifepristone after a telehealth appointment , rather than an in - person physician ’s visit , andthey can then receive it in someone from a pharmacyor by post .
Telehealth abortions are as safe and effectiveas those prescribe in someone , but in a small per centum of cases , masses may experience serious side effects or require extra treatment to terminate their pregnancy . The plaintiffs argued that , if tasked with treating such patients , AHM member would have to divert resources from other patients , and in plus , treating patient role with these side result would violate the doctors ' moral position around miscarriage .
associate : Roe v. Wade : Facts about the turning point case

The Supreme Court had to handle whether AHM had the sound standing to process the FDA on these earth . On Thursday ( June 13 ) , the court nemine contradicente concord that the plaintiff did not have sound standing .
— Abortion laws by land : https://reproductiverights.org / function / miscarriage - laws - by - state/
— For head about sound rights and self - managed miscarriage : www.reprolegalhelpline.org

— To find an abortion clinic in the U.S.:www.ineedanA.com
— Miscarriage & Abortion Hotline mesh by doctors who can offer expert medical advice : Available onlineor at 833 - 246 - 2632
— To find practical support accessing abortion : www.apiarycollective.org
In a 9 - 0 opinion save by Justice Brett Kavanaugh , the courtyard declared that " the plaintiffs have fail to demonstrate that FDA ’s relaxed regulatory requisite in all likelihood would cause them to suffer an accidental injury in fact . For that reason , the federal court are the wrong forum for addressing the plaintiffs ' business about FDA ’s action . "
The full text of the rulingcan be read online .
The regimen ask mifepristone and a 2d pill , misoprostol , is the most normally prescribed regimen for medication abortions in the United States . Mifepristone finish up in the Supreme Court after its commendation and regulation was challenged in lower courts .

ab initio , aTexas judge ruledthat the FDA ’s original approval of the contraceptive pill in 2000 should be overturned . That ruling was appealed , andan appellate court saidthat the drug ’s approval should stand unchallenged .
— 8 Supreme Court decisiveness that alter US family
— Are ' home cure ' for miscarriage safe ? ( expert say no . )

— metro - tying surgical process and vasectomies skyrocket post - hard roe
However , the appellate court want to wheel back regulatory change that the FDA made in 2016 and 2021 , on the grounds that the FDA did n’t adequately keep an eye on procedure or explain its abstract thought when it made these changes . This rollback would have reinstate the requirement for patients to get the lozenge in person from a certified doctor , along with other limitation .
The appellate court ’s decision was then appealed by both the AHM and FDA , and the case moved up to SCOTUS .

Had SCOTUS determine that the AHM had effectual standing , it could have impacted the FDA ’s dominance over all drug — not only miscarriage pills . stakeholder in the pharmaceutic industryandmedical ethical code expertsexpressed concerns that setting such a precedent could have opened the room access for doctors to action over any drug favourable reception or regularization they disagree with , disregardless of that medicine ’s prophylactic or effectivity .
Ever inquire whysome people build muscle more easy than othersorwhy freckles get out in the sun ? send out us your motion about how the human trunk works tocommunity@livescience.comwith the subject occupation " Health Desk Q , " and you may see your question answered on the web site !