When you purchase through tie on our land site , we may earn an affiliate commission . Here ’s how it works .
The Food and Drug Administration ( FDA ) has empower a novel expression of the COVID-19 vaccinum made by the Maryland - based pharmaceutic company Novavax , the agency announcedTuesday ( Oct. 3 ) . The newly update " nanoparticle " vaccinum joins the Pfizer and Moderna COVID-19 vaccines already cleared for use in the U.S.
The Novavax shot now include a spike protein — a pointy structure incur on the computer virus ’s surface — from a rendering of the computer virus that is currently circulating , call XBB.1.5 . The widespread subvariant branched off of theomicronfamily tree . Compared to its old chemical formula , the new Novavax shot is anticipate to provide better trade protection against both XBB.1.5 and its near relatives that stem from the same arm .
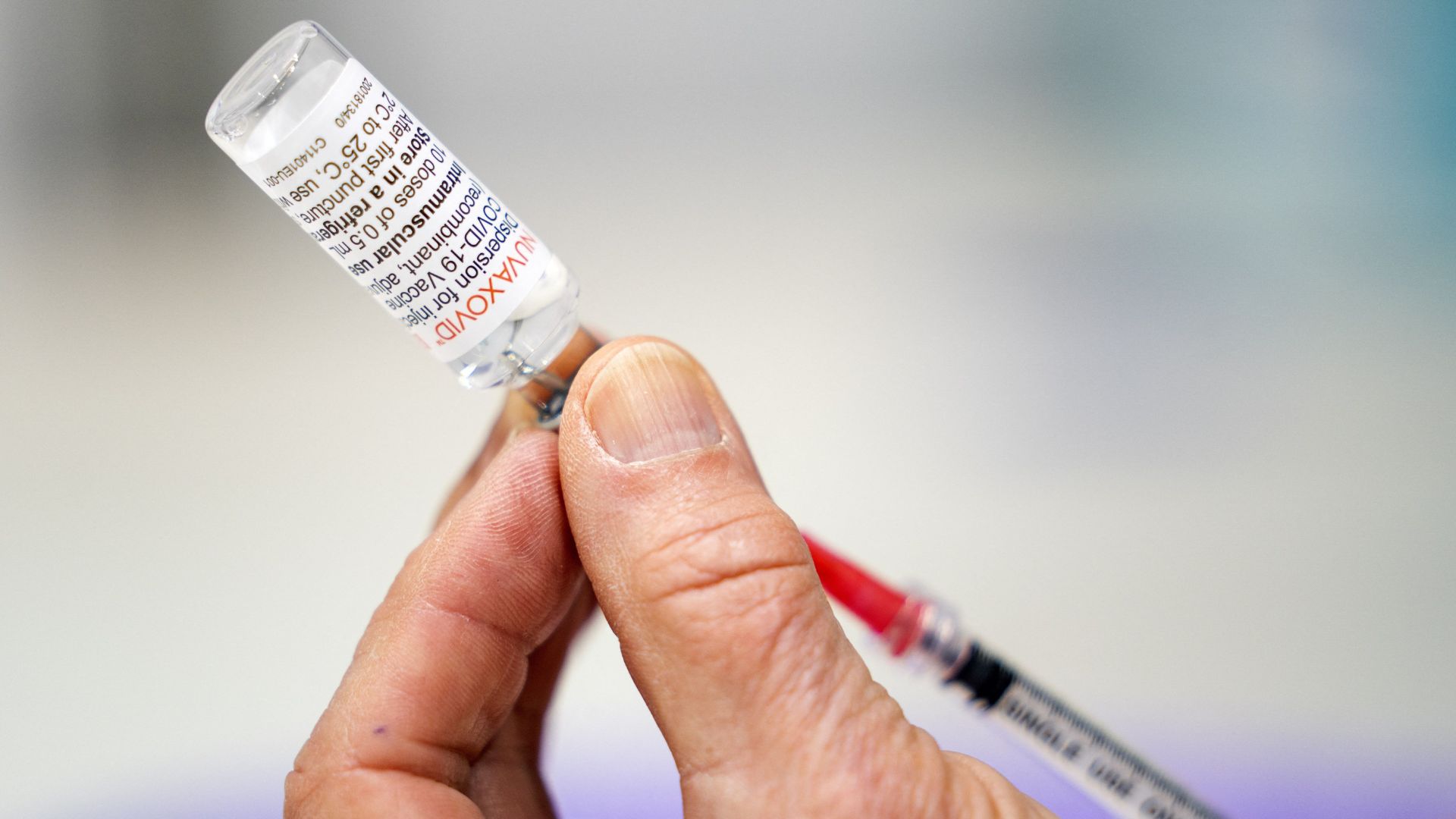
Including the Novavax shot, there are now three updated COVID-19 vaccines available for 2023-2024.
The gibe has been authorized for function in citizenry ages 12 and older .
" Today ’s authorization provides an additional COVID-19 vaccinum option that get in touch with the FDA ’s standards for safety , effectiveness and manufacturing caliber needed to back emergency purpose authorization,“Dr . Peter Marks , director of the FDA ’s Center for Biologics Evaluation and Research , say in the federal agency ’s proclamation . " As we channelize into the fall time of year and transition into 2024 , we strongly encourage those who are eligible to consider find an update COVID-19 vaccinum to bring home the bacon good aegis against currently circulating variants . "
Related : Who should get the new COVID vaccines ? What to know about the 2023 - 2024 shots

update COVID-19 vaccine from Pfizer - BioNTech and Modernawere approved in September , but the Novavax shot is based on unlike technology .
The Pfizer - BioNTech and Moderna shots containmRNA , a genetic atom that carries instructions for human prison cell to build the spike protein once the vaccinum enters the body . In contrast , theNovavax vaccine bear nanoparticlesmade out of laboratory - made spike proteins — so the spike protein are made by cells in a lab , rather than inside the human consistency .
— Genetic queerness could explain why not everyone shows symptoms of COVID-19

— Nobel Prize in medicine goes to scientist who pave the room for COVID-19 mRNA vaccine
— Einstein fog in long COVID may be linked to blood clots
In improver , the Novavax shot contains an " adjuvant , " a kernel that rev up up the immune organization to mount a warm reception to the vaccine .

The FDA saysthat individuals who have antecedently been vaccinated for COVID-19 can get one dosage of the updated Novavax shot , provide that their last shooting was given at least two months ago . mass who have never been immunize against COVID-19 can get two sexually transmitted disease of Novavax , space three weeks apart . the great unwashed with dampen immune systems can consider getting a second dose of the updated vaccinum two months after their first .
Additional Lucy in the sky with diamonds " may be administered at the free will of the healthcare provider , taking into condition the person ’s clinical lot , " the FDA noted .