When you purchase through links on our site , we may earn an affiliate commissioning . Here ’s how it work .
A key ingredient in popular decongestant such as Sudafed PE , Benadryl Allergy Plus Congestion and DayQuil Cold & Flu does n’t relieve adenoidal congestion when consider orally , a Food and Drug Administration ( FDA ) gore conclude in a meeting Tuesday ( Sept. 12 ) .
After critique years ' worth of data , the FDA ’s Nonprescription Drug Advisory Committee ( NDAC ) find that the effectiveness of the decongestant ingredient , phenylephrine , can serve relieve a stuffy olfactory organ when deliver straight into the nose — via a nasal spraying , for example — but does n’t work when look at by mouth , the 16 panelists unanimously decided .
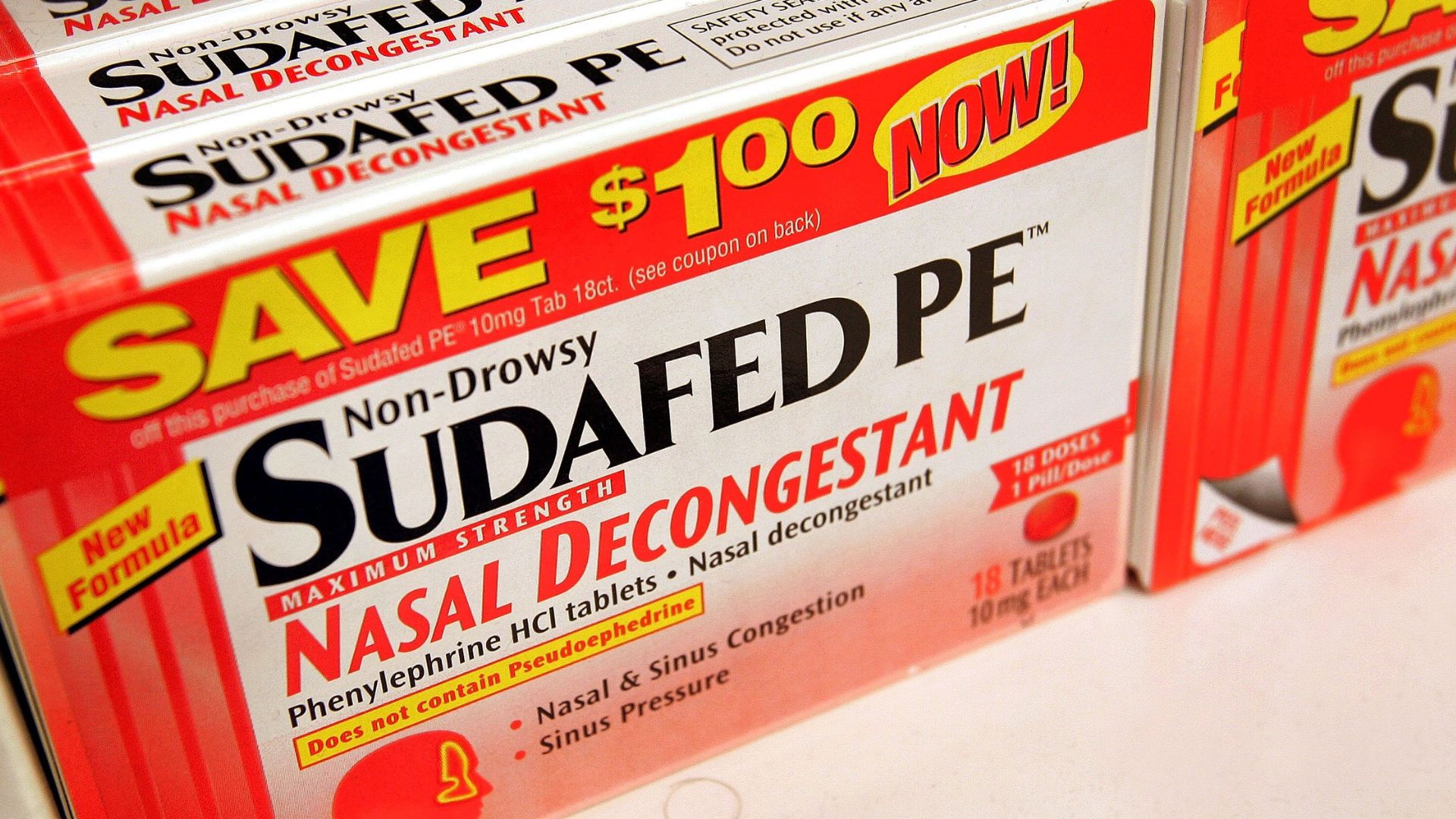
Phenylephrine, found in many over-the-counter decongestants in the U.S., doesn’t work when taken orally.
That ’s because , when choose orally , less than 1 % of the drug really end up in the bloodstream and thus get hold of the tissues of the nose that it ’s supposed to help , the committee reported in a memofrom the meeting . ( Phenylephrine is suppose to bring by constricting blood vessels in the olfactory organ and sinuses . )
Related : What are pep pill ?
So , if they do n’t work , how did phenylephrine - base birth control pill get approve in the first place ?

" The bottom transmission line is that none of the original studies stand up to New standards of study design or conduct,“Dr . Peter Starke , an FDA official who led the recap , toldThe Associated Press . Past studies of the drug had inconsistent results and too - belittled sampling sizes , and they bank on outdated statistical method and engineering that the regulators would no longer accept , Starke and his colleagues conclude .
Phenylephrine was first evaluated as an over - the - counter oral and intranasal decongestant back in 1976 , grant to the NDAC memo . But the ingredient gained popularity in 2005 as a second-stringer for pseudoephedrine , a different decongestant that had been actuate behind the counter by a law intend to harness in the sales event of drugs that can be used to make meth .
Because of this , phenylephrine soon became commonplace in over - the - counter decongestant , and now , it ’s the most democratic decongestant in the U.S.,NBC reported . Despite its popularity , though , the ingredient ’s effectivity has long been debated .

— FDA approves 1st anovulant made from human poop
— Why do n’t we breathe equally out of both nostrils ?
— Do n’t apply ' amniotic fluid ' eye drop , FDA warns

In 2007 , after newfangled formulation of popular decongestant startle rolling out , University of Florida researcher petitioned the FDAto limited review the drug ’s effectiveness in adults . The researchers allow for some grounds that the unwritten convention were ineffective , but the FDA advisers responded by saying they still needed more data point . Since 2007 , three big clinical tribulation of unwritten phenylephrine have been guide .
" These three trials represent by far the tumid and most carefully constructed trials that have ever been performed to evaluate the decongestant effect of oral PE [ phenylephrine ] , " the NDAC memoranda states . The trials showed that the drug had no more effect than a placebo , and additional data from the FDA ’s clinical pharmacology lab showed very little of it enters the blood stream .
" We consider that these raw clinical materia medica and clinical data are ordered , strong , and believable , and they sustain that orally administered PE is not effective at any dose that can be developed and still offer a reasonable gross profit margin of rubber , " the NDAC stated .

With the NDAC ’s valuation done , the FDA must now decide whether to revoke phenylephrine ’s designation as " generally recognized as dependable and effective . " If it loses that denomination , over - the - counter products containing the drug would belike call for to be removed from shelves and reformulated by suppliers , according to NBC .