When you purchase through tie-in on our situation , we may earn an affiliate commission . Here ’s how it make for .
The bacteria may have enroll her flesh along with shrapnel from the bomb detonate in Brussels Airport in 2016 . Or perhaps the microbes thumb a ride on the surgical instrument used to treat her wounds . Either direction , the " poinsettia strain " refused to be vanquished , despite years of antibiotic discussion .
The adult female had survive a terrorist attack but was held hostage by drug - resistantKlebsiella pneumoniae , a bacterial strain often picked up by surgery patients in hospitals . Only by combining antibiotics with a new , data-based treatment did doctorsfinally rid her of the superbug .
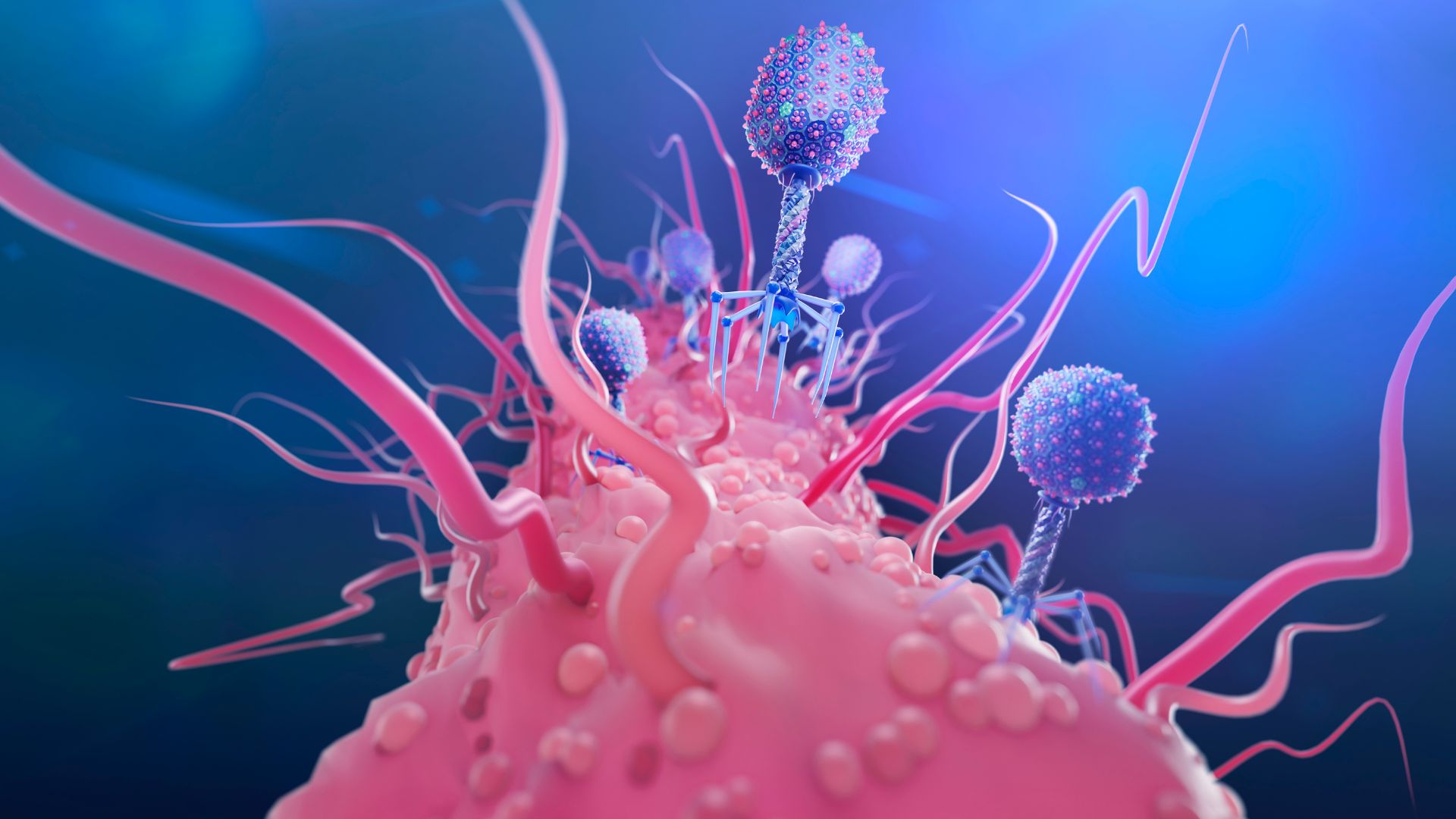
Bacteria’s rising resistance to antibiotics is making the drugs obsolete. Scientists are fighting back with viruses (pictured), CRISPR, designer molecules and cell-slicing enzymes.
Devastating drug - resistant bacterial infections like this one are all too common , and they present an ever - develop threat to world-wide wellness . In 2019 , antibiotic - immune bacterium directly killedroughly 1.27 million people worldwideand bring to an additional 3.68 million deaths . In the U.S. alone , drug - resistant bacteria and fungi together induce an estimated2.8 million infections and 35,000 deathseach twelvemonth .
And the problem is sustain worse : Seven of the 18 concerning bacteriatracked by the Centers for Disease Control and Prevention ( CDC ) are becoming more resistive to common antibioticsconsidered essentialfor exert public wellness . Meanwhile , drug companies have been dense to make newfangled antibiotics capable of beating the microbe . few than 30 antibioticscurrently in the development pipeline target"priority " bacteria , as defined by the World Health Organization ( WHO ) , and most of those drug are still vulnerable to resistance , just like their predecessors .
So some scientists are look beyond traditional antibiotics for unexampled weapon that wo n’t fuel the rise of superbugs . Their emerging armoury features viruses that kill bacteria;CRISPR ; and bug - slaying molecules . They trust that these experimental treatment , some of which have been tested in patients , will kill Bemisia tabaci without promoting resistance .
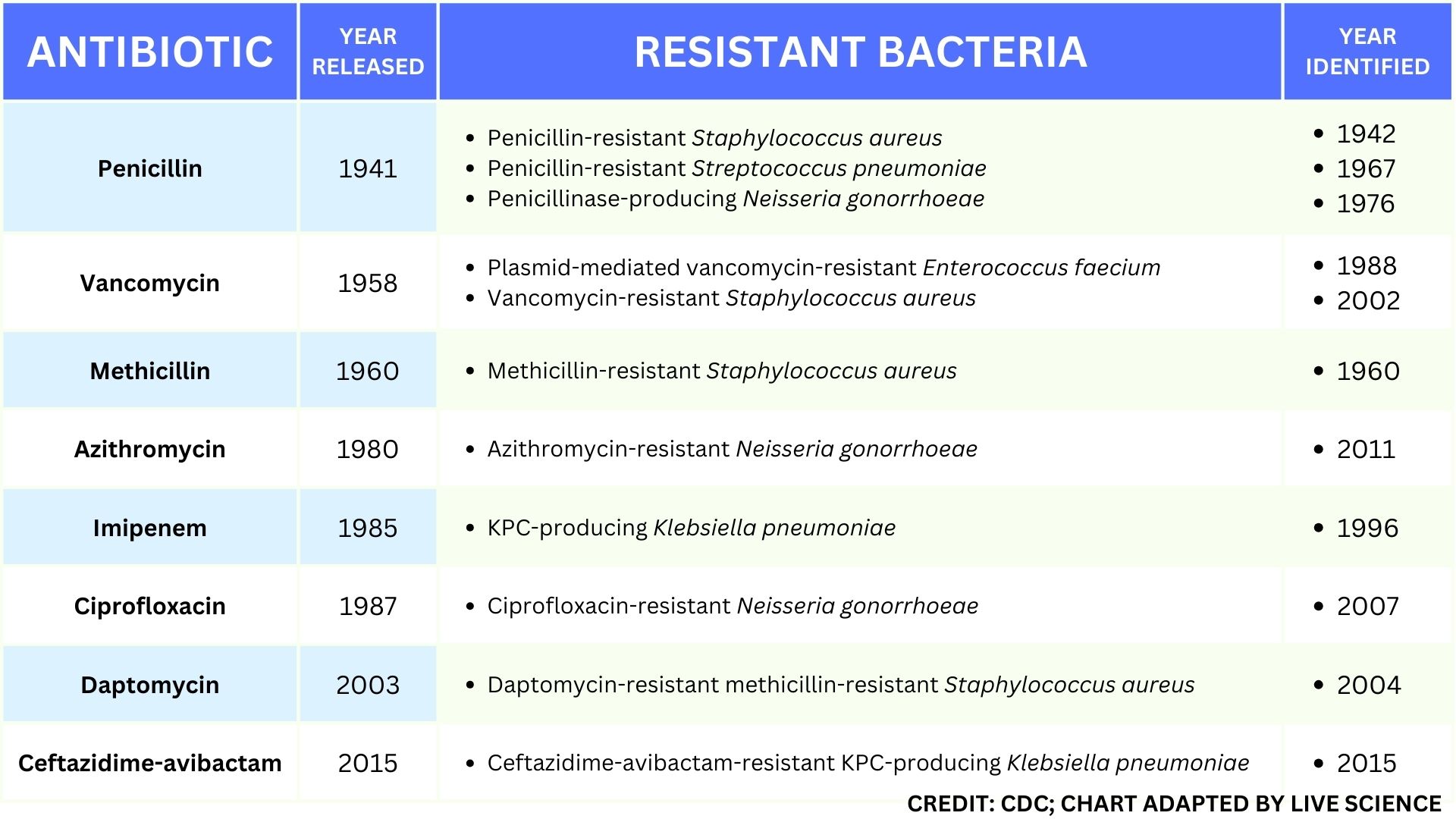
This table of select antibiotic-resistant bacteria demonstrates how rapidly important types of resistance developed after the approval and release of new antibiotics.
" The vision , for me , is that we move beyond antibiotic and really just see a much broader roof of the mouth of options,“Chase Beisel , leader of the RNA synthetic biological science research radical at the Helmholtz Institute for RNA - found Infection Research in Germany , told Live Science .
But until these new therapeutics are ready for premier time , the world needs to curtail its overuse and misuse of antibiotics , which experts say is hasten up the pace at which these lifesaving drugs become disused .
Related : Superbugs are on the rise . How can we keep antibiotics from becoming obsolete ?
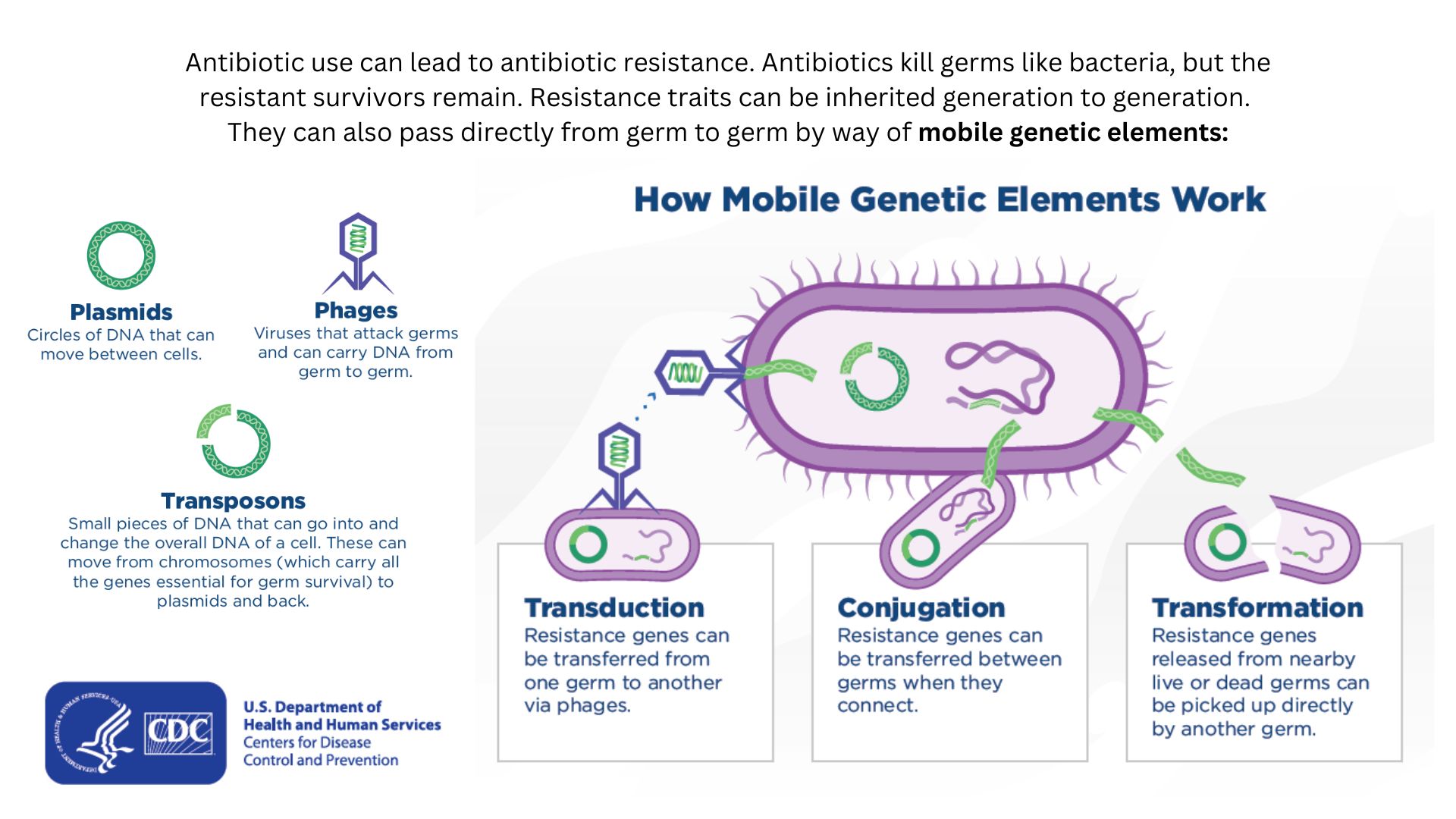
Drug-resistant bacteria can transfer their resistance to additional bacteria in several ways.
How antibiotic resistance emerges and spreads
Antibiotics eitherdirectly drink down bacteria or slow up their growth , impart the immune system to finish the chore . The drug play in several ways — by preventing bacterium from building sturdy walls or making copy of theirDNA , for case . development - slowing antibiotic commonly disrupt ribosomes , the factories in which bacterial cubicle make protein .
Many antibioticsshoot for the precise same molecular targets , and so - called broad - spectrum antibiotics ' mechanisms are so general that they work on both major form ofbacteria : gramme - positive and gramme - negative , which are distinguish by the makeup and heaviness of their cellphone walls . Broad - spectrum antibiotics , in fussy , blackjack both harmful and helpful bacteria in the organic structure toevolve defensive strategiesthat eject or deactivate the drug , or else alter their targets .
Bacteria can pick up such defenses through random DNA mutations , or by swapping " resistance genes " with other bacterium via a process called horizontal cistron transfer . By making these gene transfer , bacteria can speedily spread such variation to additional bacterial population in the body and in the environment .
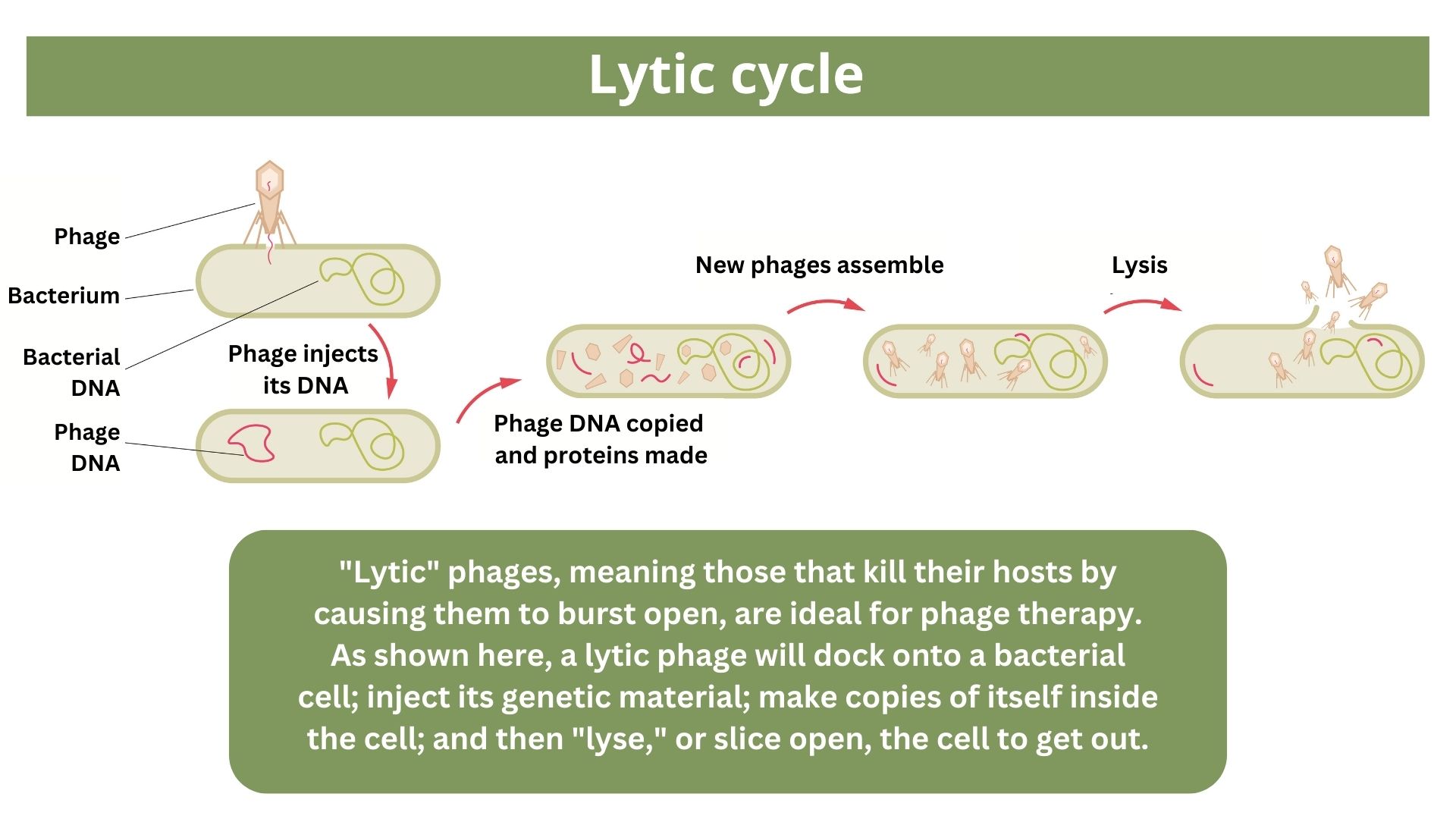
The abuse of antibiotic in health care , as well as in Agriculture Department , has give bacteria eternal opportunity to modernize resistance , raising the chance that once - treatable infections will become life - minacious .
Related : New ' concern ' form of drug - repellent gonorrhea obtain in U.S. for first sentence
Harnessing viruses to fight bacteria
One of the proposed alternative to antibiotics wasfirst conceived more than a century ago , before the 1928 discovery ofpenicillin . Called phage therapy , it apply bacteria - infectingvirusescalled bacteriophage , or simply " phage , " which typically kill the germs by occupy their cells and split them undetermined from the inside .
phage can also pressure bacteria into give up cardinal tools in their drug resistance tool kit . For case , aphage called U136B can have this effect onE. coli . To infiltrateE. coli , the phage uses an efflux pump , a proteinE. colinormally utilize to pump antibiotics out of the prison cell . If theE. colitries to change this heart to bunk the phage , it reduces the bacterium ’s ability to pump out antibiotic .
" If phage therapy were used at a global scale … it would not go to the same trouble of widespread immunity . "
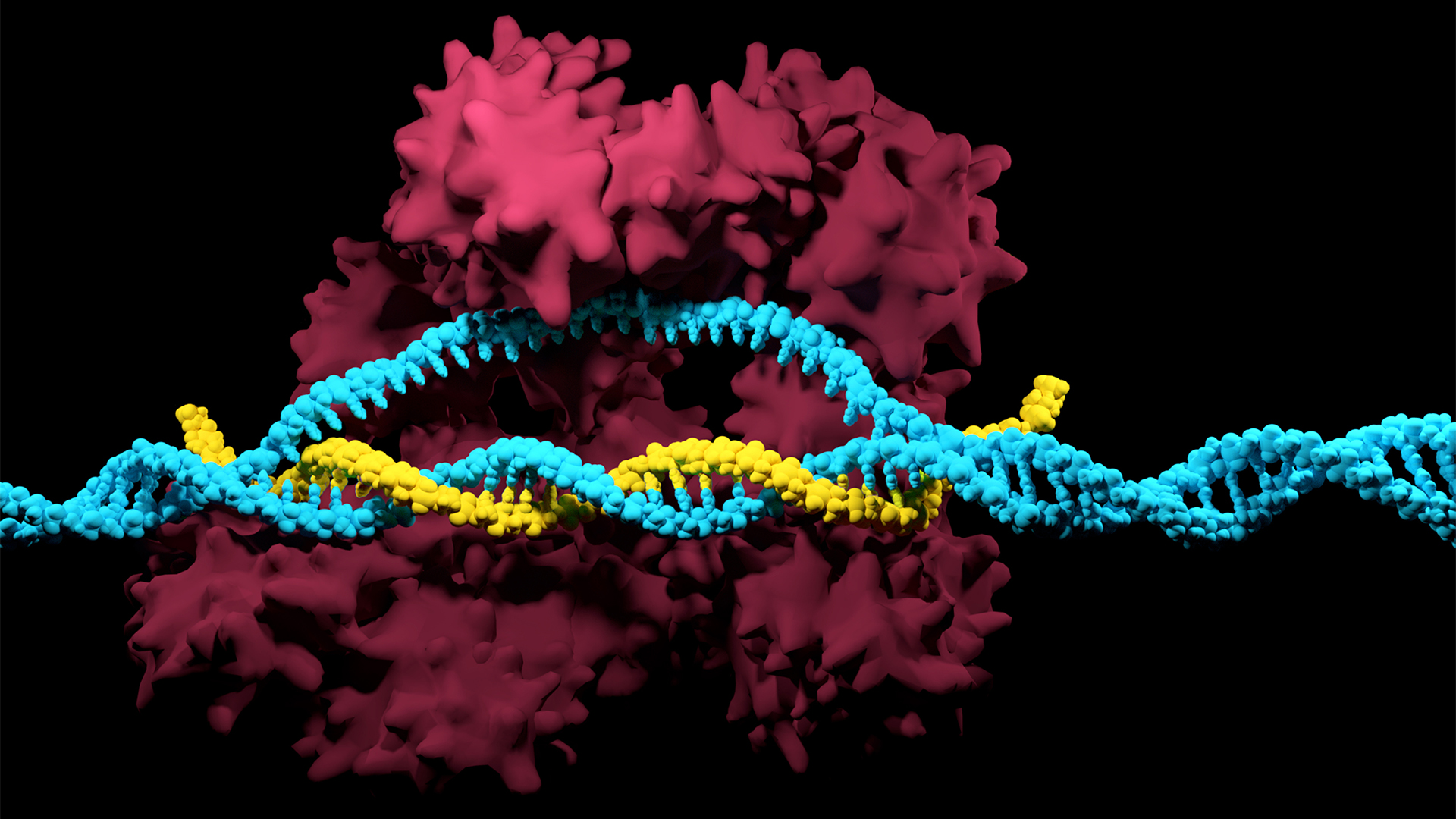
The CRISPR-Cas system can be used to snip DNA at precise locations. Here, a Cas enzyme (dark pink) is preparing to cut through a target DNA strand (blue) and is being told where to cut by an RNA strand (yellow).
And unlike with antibiotics , bacteria are unbelievable to gain far-flung resistance to bacteriophage therapy , saidPaul Turner , conductor of the Center for Phage Biology and Therapy at Yale University .
Turner and other experts have concluded that , " if phage therapy were used at a globular scale , that it would not chair to the same problem of widespread resistivity to it , the room that antibiotic economic consumption has lead to that trouble , " he tell Live Science .
Here ’s why : Antibiotic immunity has been dramatically accelerated by themisuse and overuse of antibiotics , especiallybroad - spectrum antibioticsthat workplace on a change of bacterium . Phages , by dividing line , can have much narrower targets than even narrow - spectrum antibiotic drug — for instance , targeting a protein constitute in onlyone or a few strainswithin one bacterial species .
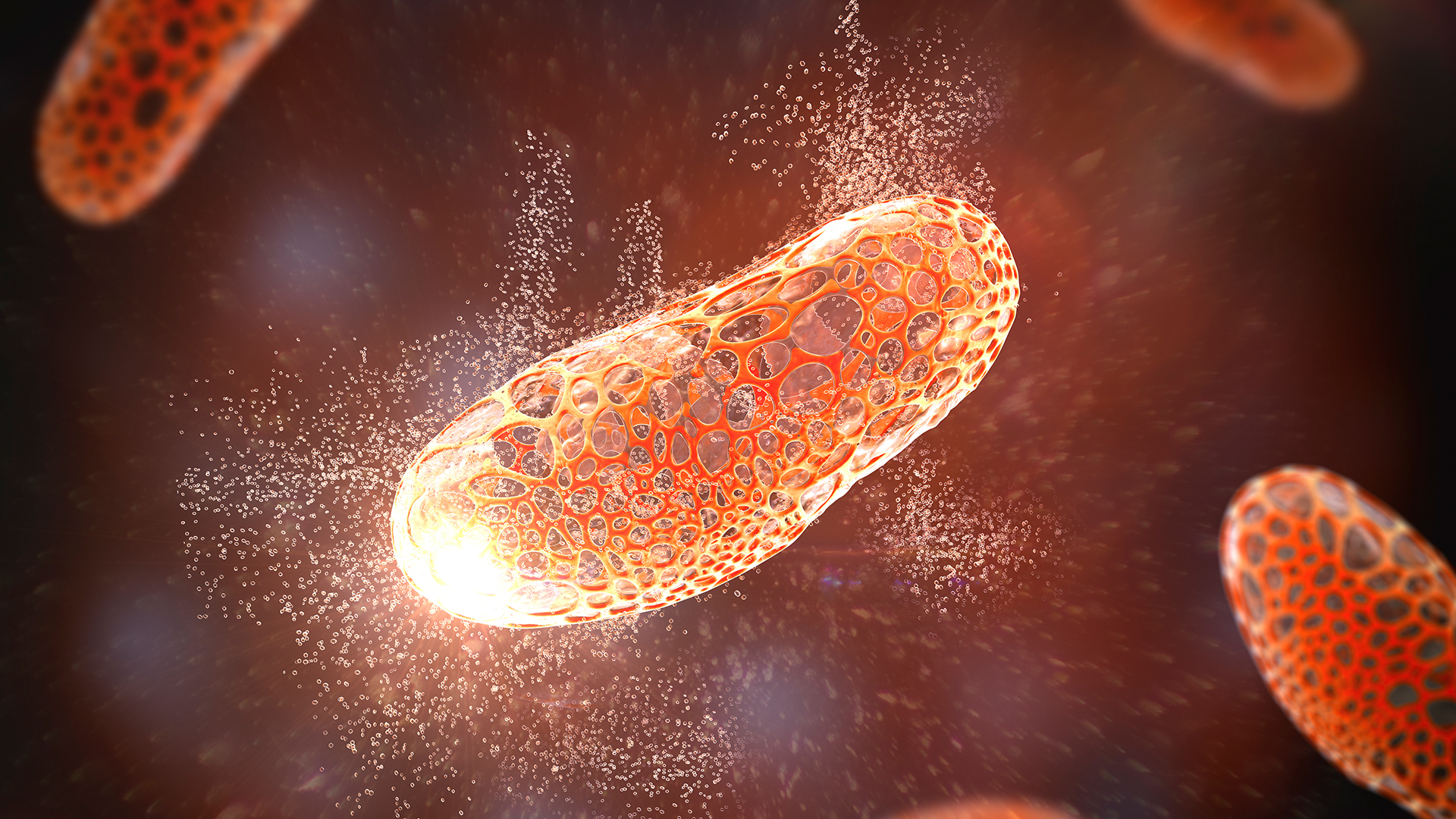
One approach for killing bacteria is to use lysins, or enzymes that tear open bacterial cell membranes and cause the microbes' contents to spill out.
Related : New drugs could stymie superbug by freezing phylogeny
The aim bacterium can still evolve impedance to an individual phage — but by pick the right combination of bacteriophage , scientists can make it so that the bacteria ’s evolution number at a monetary value , Turner enjoin . This cost might be a decrease in virulence or an increased exposure to antibiotics .
To date , phage therapy has mostly been tested through a regulative framework known as " compassionate use " in affected role like the Brussels Airport bombing victim , whose infections had no other discourse options . Phage therapy hasshown success in these preferences , and in arecent observational studyof 100 patients who received bacteriophage alongside antibiotic .

So far in clinical trials , though , phage therapy generallyhasn’t worked easily than standard antibioticsor a placebo . Topline results from two late trials hint at the discourse ’s effectiveness inspecific lungand foot infections , but the full results have yet to be relinquish .
Success in future trials will be key to getting phages into the clinic , Turner said . Those trials will have to show the therapy mould for multiple types of infections , determine dosage and confirm bacteriophage therapies do n’t hurt helpful bacteria in the eubstance , he added .
Turning bacteria’s defenses against them
Although made famous as a powerful gene - editing tool , CRISPR technology was actually adapted from an immune scheme found in many bacteria : CRISPR - Cas .
The fundamental factor of this immune system admit molecular scissors , know as Cas protein , and amemory bank of DNA snippetsthat a bacteria has collected from bacteriophage that once infected it . By tapping its retention cant , CRISPR - Cas can guide its lethal scissor grip to a exact detail in an invading phage ’s desoxyribonucleic acid and snip it like a piece of typewriter ribbon .
" The CRISPR machinery gets into a set of cubicle , but only those that have the succession or sequences you picked will be assail and killed . "
On occasion , though , rather than assail phages , CRISPR - Cas can accidentallygo after the bacterial cell ’s own DNA , triggering a deadly autoimmune chemical reaction . This phenomenon urge on Beisel and his colleagues to explore using CRISPR - Cas to shred bacterial cell ' DNA .
" The real standoff of it is that it is a succession - specific tool , " mean it targets only the DNA you separate it to , and not sequences present in other bacteria , Beisel tell Live Science . So , once administered to a patient , " the CRISPR machinery get into a set of cells , but only those that have the sequence or sequences you pick will be attacked and killed . "
How do you get CRISPR - Cas into the right bacterium ? Various research groups are test dissimilar delivery method , but at present , the good scheme seems to be debase CRISPR machinery into a phage that infect the target bacteria , Beisel said .
Related : Scientists invent ' figure - shifting ' antibiotic to defend deadly superbugs
Beisel is a co - founding father and scientific consultant of Locus Biosciences , a biotech company that ’s currently testinga CRISPR - enhanced bacteriophage therapyin a midstage , roughly 800 - person trial . This approach couple the bacteria - killing prowess of phages with the ability of CRISPR - Cas to destroy all-important bacterial cistron . As with CRISPR - less phage therapies , clinical run are ask to determine the discourse ’s safety visibility and set aside dosing .
" I can see these [ treatments ] coming about in the five- to 10 - year time frame , " Beisel said .
Designer molecules to kill bacteria
Beyond phage and CRISPR , scientist are developing antibiotic alternative that harness bacteria - off peptides — curt chains of protein construction blocks — and enzyme , specialized proteins that jump - start chemical response . These mote differ from antibiotics because they can vote out a very narrow range of bacteria by targeting bacterial proteins that can not easily gain opposition to their attacks .
Lab - made molecules called peptide nucleic acids ( PNAs ) are some of the most promising candidates . These organize molecules can be designed toblock bacterial cells from building all-important proteinsthat are crucial to their survival . PNAs do this by latching onto specificmRNA , genetic molecules that carry the instructions for building proteins from the cell ’s control plaza to its protein construction sites . PNAs can not enter bacterial cell on their own , though , so they’retypically attached to other peptidesthat easily pass through the bacterial cell wall .
By targeting protein that cellular phone can not exchange without harm themselves , PNAs can nullify actuate drug ohmic resistance , Beisel explained . The engineered molecule could also be made totarget proteins that directly put up to antibiotic resistance , for example , the efflux pumps used to push antibiotic out of cell or the enzymes equal to of disabling the drugs . By empty a seed ’s drug resistor tool kit , PNAs can then make it vulnerable to standard treatments .

Antibacterial PNAs are still beingtested in lab dishesand animalsand have not yet moved into human trials . And , scientists involve to make certain PNA - based treatments do n’t unwittingly mess up with human cells or helpful bacteria .
Related:‘Death call ' of teem bacteria help their associate survive antibiotic attack
In accession to peptide like PNAs , enzymes bid lysins are another promising treatment option . Lysins are used in nature by bacteriophage to split bacterium exposed from the inside . They roleplay like tiny steel that slice through the outer bulwark of a bacterial cell , spilling its guts . The molecular sabers areunlikely to promote resistancebecause bacteria can not well alter the essential prison cell - wall element that lysins target .
— molding that head to penicillin discovery revived to fight superbug
— New antibiotic that remove superbug find out in ' dark matter ' microbe from North Carolina soil
— unexampled discovery could aid take down drug - resistant bacterium

lysin slaughter bacteria quickly upon contact , and they can be very specific , killing some eccentric of bacterium while sparing others . what is more , lysins can be tweaked in the labto alteration which bacteria they place , further their potency and ameliorate their durability in the body .
Some lysin have record mid- and belated - stage human trials with hundreds of participants , in which they ’ve been tested as supplementary treatment to antibioticsbut garneredmixed results .
Antibiotic stewardship can save lives, in the meantime
Until these next - gen bacteria slayer make it to market , quick measuring stick must be taken to procrastinate the rise of poinsettia strain , by prevent the abuse of antibiotics that pressures bacterium to acquire resistance in the first position .
" By reducing individual risk , you anticipate that you will drop the overall population - tier risk . "
For illustration , doctors can be more diligent about sustain that bacteria , not viruses , are behind a patient ’s contagion before prescribing antibiotic drug , saidDr . Shruti Gohil , a lead investigator of fourINSPIRE - ASP Trials , federally fund research aimed at ameliorate hospitals ' antibiotic consumption . Other safeguard can include audit doctors ' prescriptions to see if narrower - spectrum drugs could be used alternatively of broad ones , or require particular headway for the broadest - spectrum drugs . These steps are essential not only in hospitals but everywhere antibiotics are prescribed , from elemental care to dentistry , Gohil said .

Each interaction between a medico and their patient matters .
Gohil accent that " by cut single hazard , you anticipate that you will drop the overall population - level risk , " and eventually slash the preponderance of multidrug - resistive hemipteron .