When you buy through links on our site , we may garner an affiliate commission . Here ’s how it works .
Bismuth is an unusual ingredient that we do n’t encounter much in workaday life . But this pretty , iridescent metal , launch near the bottom of theperiodic table , display some over-the-top properties . Magnetic levitation — bismuth ’s power to seemingly be adrift between two attractor — is perhaps one of the most interesting . The revulsion between atomic number 83 and the attracter is so stiff , it do the metal to levitate .
But why isbismuthso powerfully repulse from attractor ?

Bismuth is an iridescent metal that can seemingly float between two magnets, a phenomenon known as “magnetic levitation."
harmonize toEric Riesel , a magnetized cloth apothecary at MIT , the answer comes down to the type of magnetism show by bismuth . Every material has magnetic properties , determined by a quantum property of the element ’s electron have it off as spin . But , this twist can only bespeak in two guidance — up or down — and the combination of all the spin in a material define exactly what type of magnetism the constituent will exhibit .
" Most people are familiar with ferromagnets ( permanent attractive feature ) like Fe , where the spins are all aligned with each other , but there are also anti - ferromagnets where the spins are pointed in opposite directions to each other , " Riesel told Live Science .
However , there ’s also another pair of charismatic categories : paramagnetism and diamagnetism . " In paramagnets , when you apply a magnetized field , spins in that material will align with the field of view in proportionality to its strength , " he allege . " diamagnet give a strength in the opposite focal point to the field of force , repelling it . "
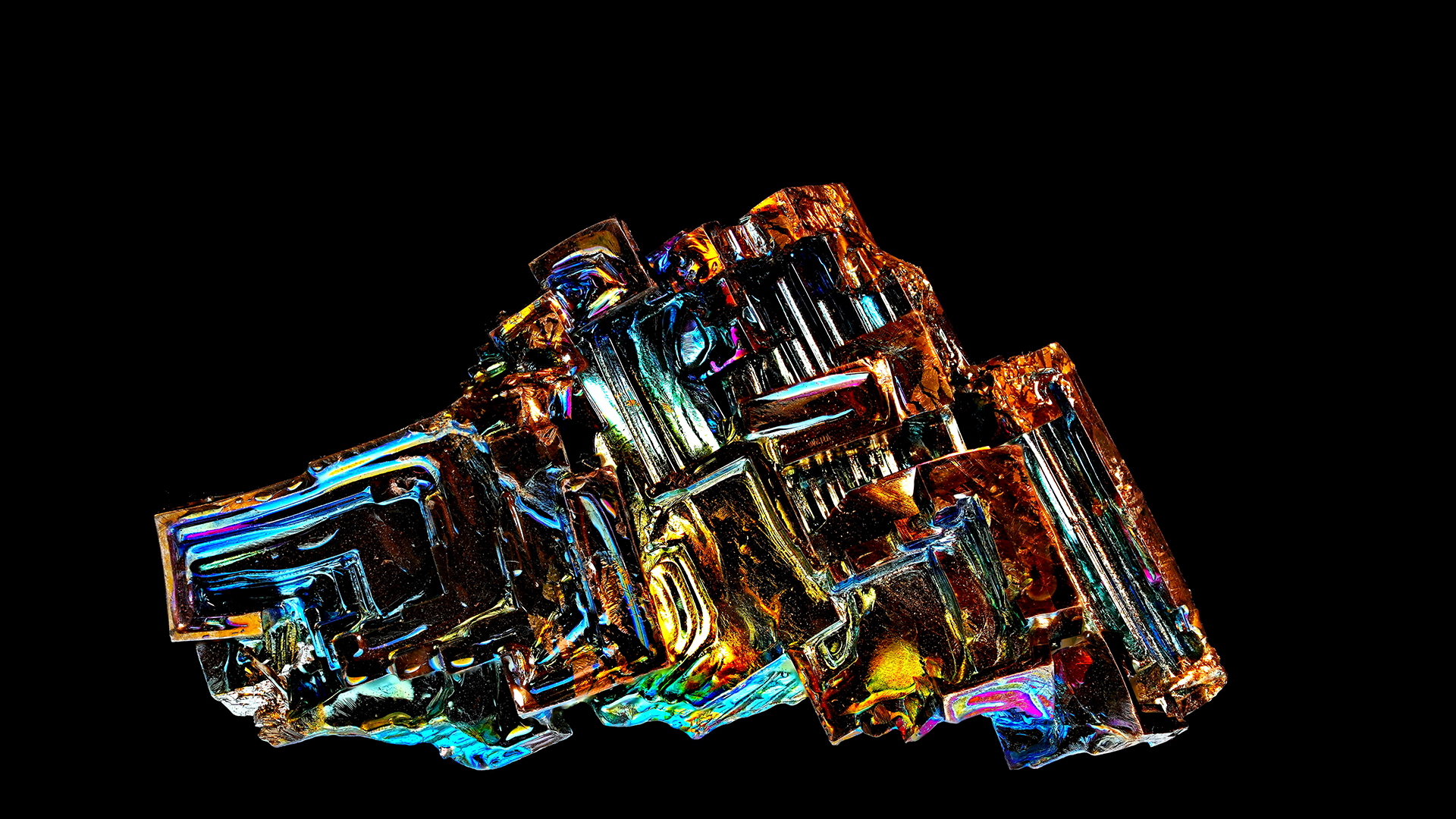
Bismuth is an iridescent metal that can seemingly float between two magnets, a phenomenon known as “magnetic levitation."
bear on : Is it possible to reach out-and-out zero ?
Bismuth is an exercise of a diamagnetic stuff , yet this is not the behavior we would expect from the element ’s electron configuration . The eccentric of magnetism demonstrate by a textile depends on the arrangement of electrons and their corresponding spin . negatron encircle the nucleus in defined layers called shells , which are further subdivide into levels called the s , d , p and f orbitals .
Typically , diamagnetic materials have a closed shell body structure . This means a particular group of orbitals are completely full and the electrons have been forced to pair , with one pointing up and the other down — essentially scratch out the spin . Conversely , paramagnetic fabric usually have part replete orbitals , mean the electrons are unpaired and can align their spins in the same direction .
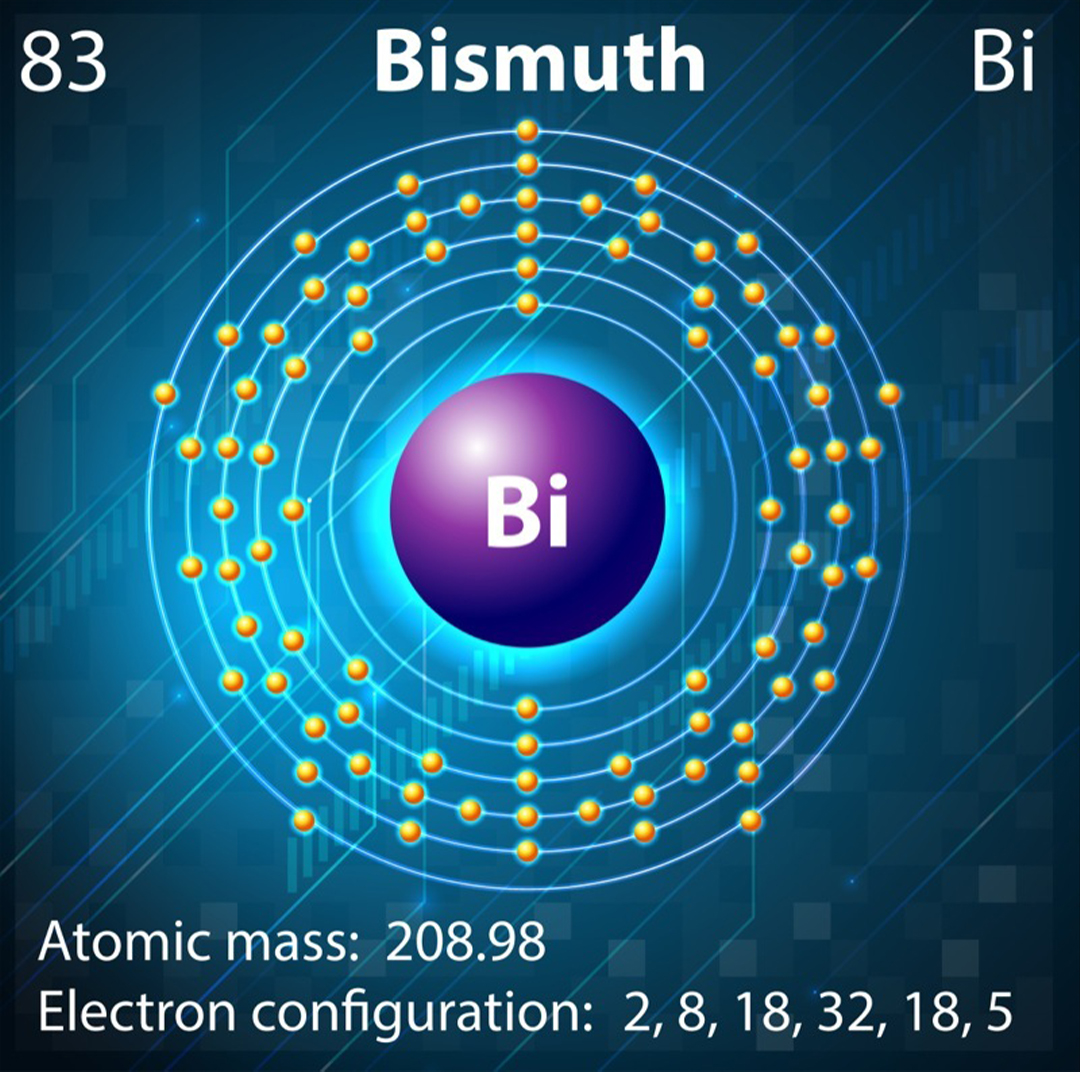
The unfilled outer shell of bismuth means it should be weakly attracted to magnets but, relativistic heavy atom effects mean we can’t predict bismuth’s magnetic properties from just its electron configuration.
Bismuth is in Group 15 of the periodic board . The s , ergocalciferol and f orbitals are all full , but the phosphorus orbitals curb three out of a potential six electrons . So bismuth has partially filled orbitals and should deport as a paramagnet . However , its office in row six of the periodic table means Bi also possess some strange heavy - atom properties .
" Chemical element found after the f - block in the periodic tabular array have their outermost electrons orb the nucleus at speeds that are significant fraction of the speed of lightness , " saidIra Martyniak , also a magnetic materials chemist at MIT . " The direct relativistic outcome micturate the 6s and 6p orbitals contract and reside closer to the nucleus , which gives ascent to anomalous physical and chemical characteristics . "
These relativistic result are creditworthy for many of atomic number 83 ’s surprising properties , such as itsunconventional superconductivity , its very low thaw point ( 520.7 degrees Fahrenheit , or 271.5 degree Celsius ) and the strange configuration of its watch glass . The unexpected diamagnetism is no exclusion .

— Is atomic number 1 a metallic element ?
— Is glass a liquid or a satisfying ?
— What ’s the fastest thing on Earth ?

" Even though atomic number 83 has the unmated negatron in its 6p orbital , because of relativistic condensation of the 6s and 6p floor , the paramagnetism stem from the 6p electrons is inhibit and the behavior of bismuth is mostly overshadow by the closed shell and big size of it of the mote , extend to strong diamagnetism , " Martyniak told Live Science .
Diamagnetic material have lots of worthful covering , includingelectromagnetic evocation in Cu coils(used to generate electricity ) and thealuminum caterpillar tread of gamy - stop number maglev trains . Bismuth itself is too sonorous to be a practical textile for worldwide exercise , but its potent diamagnetism means it is now a common component insuperconductorsandquantum computing .
scientist create ultra - tough pig alloy that is stronger than sword and can withstand temperature of 1500 F

Why does nearly all lifespan breathe O ?
The ceaseless surveillance of New life could worsen our brain use in style we do n’t amply understand , disturbing survey evoke






