When you purchase through link on our web site , we may garner an affiliate commissioning . Here ’s how it work .
In a historical first , the Food and Drug Administration ( FDA ) has approve America ’s firstgene therapiesfor sickle - mobile phone disease ( SCD ) , one of which habituate the gene - editing toolCRISPR .
" Sickle cell disease is a rare , drain and animation - jeopardise rakehell disorder with significant unmet need [ for better , long - lasting treatments ] , and we are activated to pass on the field especially for individuals whose lives have been seriously disrupted by the disease by sanction two cell - based factor therapies today,“Dr . Nicole Verdun , film director of the Office of Therapeutic Products within the FDA ’s Center for Biologics Evaluation and Research , said in astatement released Friday(Dec . 8) .
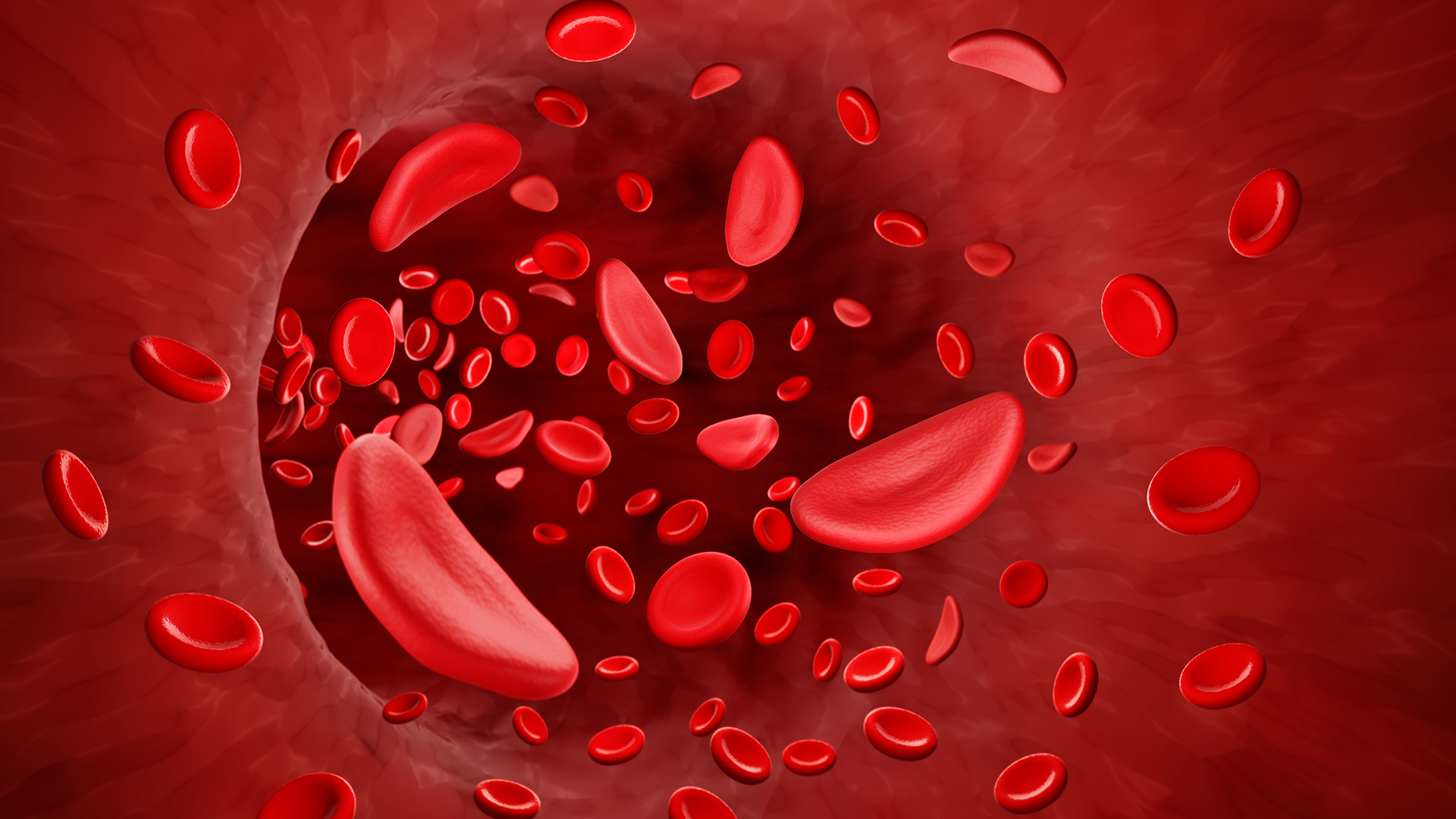
Sickle-cell disease causes red blood cells to become C-shaped.
" Gene therapy prevail the promise of delivering more targeted and in force intervention , specially for somebody with rare diseases where the current discussion choice are modified , " she tell .
The U.K. became thefirst nation to approve the CRISPR - base therapy , foretell Casgevy , in mid - November . Experts anticipated that the FDA would before long echo the decision made by U.K. regulators , as advisors to the FDA had deemed the treatmentsafe for clinical utilization back in October .
connect : The human race ’s first CRISPR therapy has just been okay . Here ’s everything you need to know

SCD iscaused by genetic mutationsthat change the shape of the protein hemoglobin , which carries oxygen in flushed lineage prison cell . violent rip cells then become sickle - shaped , rather than round , which causes them to break off apace and also stick together , blocking rip vessel .
The CRISPR - base therapy Casgevy stops the sickling of cells by switching off a cistron name BCL11A.
The CRISPR system can precisely guide a pair of molecular scissors to the gene doctors want to disable and then cut that gene out of a mortal ’s desoxyribonucleic acid . Disabling the BCL11A cistron make it so a patient ’s cells can make a variation of hemoglobin normally made only in the womb . Only the adult adaptation of hemoglobin is affected in SCD , so enabling the body to make this foetal Hb reverse the patient ’s anaemia .

" In patient role with sickle cell disease , increased levels of HbF [ foetal hemoglobin ] keep the sickling of red blood cells , " the FDA stated .
To apply the treatment , doctors first draw a patient ’s roue stem cells — unspecialized cadre that can transubstantiate into dissimilar jail cell in the stemma . They then edit the cells to invalid the BCL11A gene and return them to the patient ’s body . Before the infusion , the patient role must take a chemotherapy drug to eliminate the unedited root word cells still in their bone marrow .
The second cistron therapy approve by the FDA , called Lyfgenia , does not use CRISPR . rather , the treatment uses a harmless computer virus , call in a lentiviral vector , to deliver new deoxyribonucleic acid into patients ' blood root word cells .

The virus inserts a functional Hb gene to supplant patients ' mutant one . The functional gene makes a version of Hb that ’s very exchangeable to that seen in adult without SCD , but it has additional place that aid limit the sickling of blood cells . blood line cells change cast in SCD when the abnormal hemoglobin clumps together , or " polymerizes , " to organize firm chains within the cells . The tweaked hemoglobin used in Lyfgenia ismore tolerant to that polymerizationthan normal hemoglobin , due to its bodily structure .
As with Casgevy , patients take a chemotherapy drug before receive their new cadre treated with Lyfgenia .
Casgevy is approved for SCD patient ages 12 and older with " recurrent vaso - occlusive crises , " meaning events where sickled red ancestry cells block off the atomic number 8 flow into pipe organ , causing tissue paper terms and severe pain . Lyfgenia is okay for patient ages 12 and older with a history of vaso - occlusive events , the broad class of complications that crises fall under .

— Could CRISPR bring around HIV someday ?
— CRISPR therapy for high cholesterin shows hope in early trial
— New gene therapy gel is the 1st approved treatment for rarified and dreadful ' butterfly stroke disease '
" Today ’s action follow strict evaluations of the scientific and clinical data needed to fend for approval , shine the FDA ’s commitment to facilitate developing of dependable and efficacious treatment for conditions with wicked impacts on human health,“Dr . Peter Marks , director of the FDA ’s Center for Biologics Evaluation and Research , said in the FDA argument .
Ever wonder whysome people establish heftiness more easily than othersorwhy lentigo come out in the sun ? Send us your questions about how the human soundbox figure out tocommunity@livescience.comwith the subject line " Health Desk Q , " and you may see your doubtfulness answered on the website !